Summary
- Mustard proteins exhibit excellent fat and water binding properties, plus gelling and emulsion stabilizing properties
- Applications include meat emulsions, carbonated beverages, and fortification of pasta products
- Protein content of mustard seeds is ~28-32% and mustard flour in mid 40%
- Mustard is an excellent plant protein source with good essential amino acid profile; rich in lysine, adequate amounts of methionine & cysteine that can complement cereal proteins
- Cruciferin and napin, the seed storage proteins in mustard, have different molecular structures, amino acid profiles, and unique biological, physico-chemical, and functional properties
The demand for plant-based proteins is steadily growing due to rising middle-class incomes globally, increased interest in health and nutrition, and concern for animal welfare and the environment. Plant proteins are now being incorporated into processed foods, energy drinks, sport nutrition products and dietary supplements for their functional attributes and nutritional benefits.
While soy is the dominant plant protein in the global market, emerging proteins such as wheat and pea are having significant growth. Mustard, too, can capture a slice of this growing market particularly in the specialty sector since its high protein content with good amino acid profile make it an attractive source of food-grade vegetable protein.
Mustard Composition
Oriental and Brown mustard (Brassica juncea) and Yellow (White) mustard (Sinapis alba) are most commonly used as condiments and flavouring agents in North America and Europe. Processed mustards (e.g. ground seeds, flours, meals, and bran) are used as functional ingredients in salad dressings, mayonnaise, sauces, pickles and processed meats. In many regions of Asia, the extracted oil from B. juncea is used as cooking oil.
Mustard seeds are comprised of protein, oil, and carbohydrates as well as the minor constituents including minerals (4%), glucosinolates (100-200 μmoles/g) and phytate (2%) (Figure 1). Yellow mustard contains about 5.5% mucilage in the outer seed coat compared to the negligible amounts in Brown or Oriental mustards.
There is growing interest in developing viable commercial processes to extract and isolate mustard proteins, characterize the composition and functionality to find high value applications in human and animal nutrition.
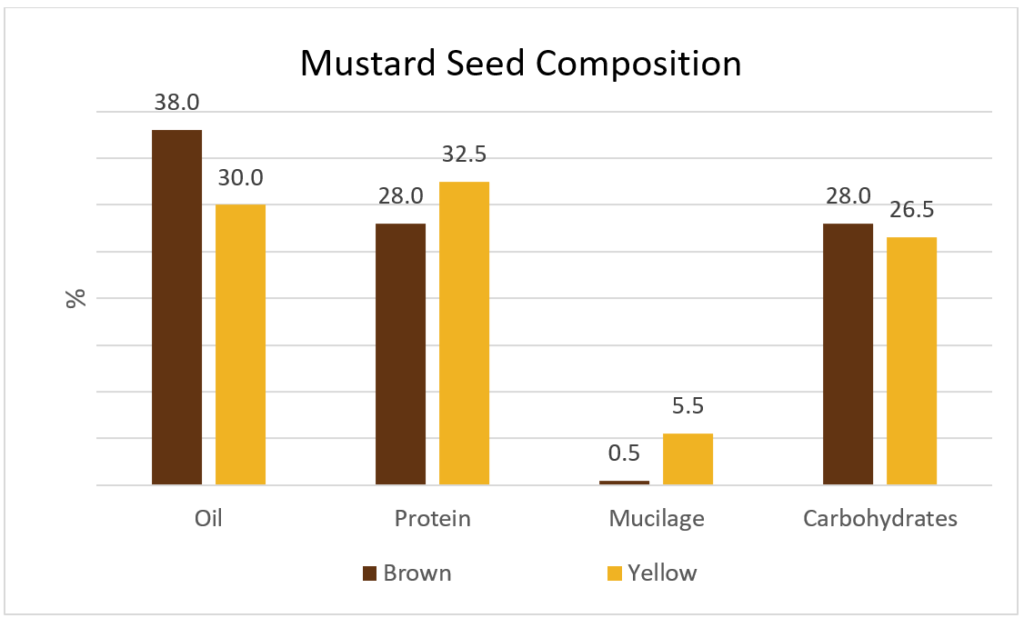
Mustard has an excellent nutritional profile being rich in lysine with adequate amounts of sulphur-containing amino acids (methionine & cysteine) which are limiting amino acids in most cereal proteins13 (Table 1). Due to the high biological value, mustard protein could be of great interest to human nutrition.5
Table 1. Comparison of amino acid profile of Yellow mustard (YM) flour to defatted meals from YM, canola and soybean*
| Commercial defatted YM flour | YM meal12 | YM meal4 | Canola meal | Soybean meal6 | |
|---|---|---|---|---|---|
| Histidine | 18 | 12 | 19 | 16 | 24 |
| Isoleucine | 19 | 29 | 31 | 18 | 50 |
| Leucine | 32 | 38 | 57 | 33 | 80 |
| Lysine | 38 | 22 | 48 | 25 | 61 |
| Methionine | 9 | 11 | 16 | 11 | 12 |
| Phenylalanine | 17 | 21 | 32 | 19 | 50 |
| Threonine | 20 | 21 | 39 | 20 | 40 |
| Tryptophan | 7 | 8 | 15 | 7 | -c |
| Valine | 22 | 22 | 43 | 20 | 50 |
| Alanine | 23 | 27 | 34 | 21 | 44 |
| Arginine | 34 | 26 | 51 | 33 | 72 |
| Aspartic acid | 22 | 54 | 69a | 39 | 123 |
| Glutamic acid | 139 | 93 | 133b | 85 | 197 |
| Glycine | 27 | 36 | 44 | 26 | 42 |
| Proline | 48 | 32 | 48 | 26 | 53 |
| Serine | 22 | 26 | 37 | 20 | 51 |
| Cysteine +cystine | 19 | 17 | 16 | 22 | 12 |
| Tyrosine | 10 | 14 | 29 | 14 | 36 |
| aAspartic acid + asparagine; bGlutamic acid + glutamine; cNot determined *differences in results noted due to cultivar used and analytical method |
|||||
The proteins of most interest in mustard are the seed storage proteins, cruciferin and napin, found within the cotyledon. Cruciferin and napin have different molecular structures, amino acid profiles, and physico-chemical and biological properties (Table 2).13
Table 2. Mustard seed storage proteins: cruciferin and napin12
| Cruciferin | Napin | |
|---|---|---|
| Molecular Structure | 12S legumin-like globulin | 2S prolamin-super family albumin |
| Molecular Weight | High (300-360kDa) | Low (12.7-20.3 kDa) |
| Biological Activity | Nutritional value | Antimycotic/antibacterial properties11 Trypsin inhibitor activity. Allergenic properties8. High in cysteine |
| Physico-Chemical Properties | Soluble in dilute salt solutions. Good solubility over wide range of pH. Least soluble at low pH | Water soluble. Highly soluble at low pH High thermal stability (pH dependent) |
Protein Recovery Technologies
Protein isolation from Yellow or Brown/Oriental mustards begins with the defatted mustard flours or meals as starting material. The different extraction, concentration and purification processes lead to various mixtures of soluble and insoluble mustard protein concentrates, isolates or enriched protein fractions containing cruciferin or napin (Figure 2). The processes can be modified to remove and/or recover glucosinolates, phytates and phenolics, compounds which contribute to the dark colour and unacceptable organoleptic properties of protein isolates, but which may have unique applications themselves.
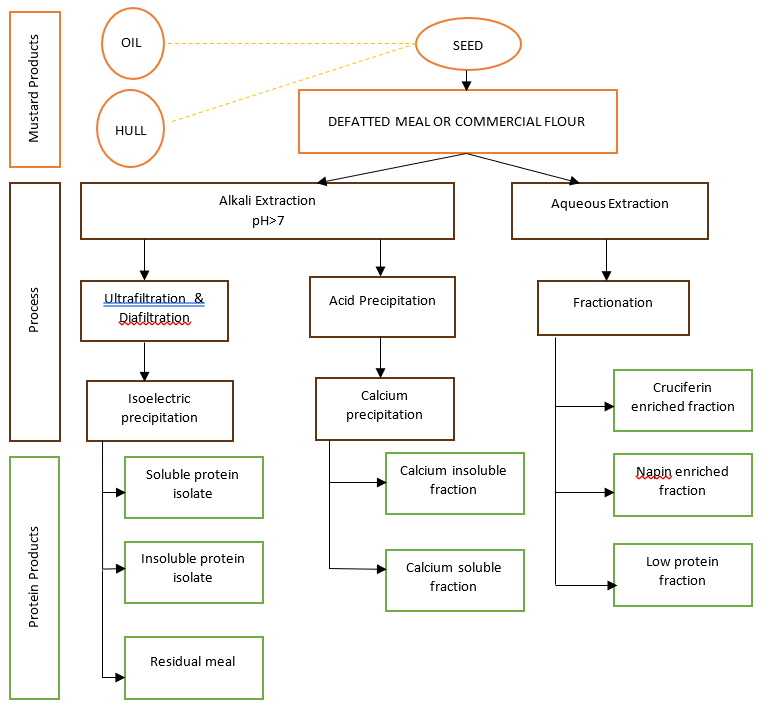
Resulting protein isolate products present opportunities to explore multiple applications since the functional properties of proteins are affected by processing, protein composition, the presence of other compounds, and the methods used to assess functionality. Protein isolates can be hydrolyzed to produce bioactive peptides or chemically modified (e.g. acetylation, phosphorylation or glycosylation) to produce proteins with different functionalities.
The key co-products such as: oil are high in erucic acid and are good candidates for use as industrial feedstock for applications such as lubricants and for biofuel; the hulls (bran) can be used as an ingredient with hulls from Yellow mustard may be used for extraction of mucilage.
Mustard Protein Applications
Mustard flour, with protein at mid 40% range, can be used as a source of protein in food formulations. This ingredient has excellent functional properties, with some notable differences between Yellow and Brown/Oriental mustard flours evident (Table 3). Brown mustard meal has a slightly higher protein content than Yellow mustard meal. Yellow mustard meal has significantly lower emulsifying ability than the Brown meal but the foaming capacity and foaming stability appear to be similar.
Table 3. Protein content and functional properties of defatted mustard meal compared to commercial soybean flour2,4
| Product | Protein (%) | PS (%) | EAI (m/g) | ES (%) | FC (%) | FS (%) |
|---|---|---|---|---|---|---|
| Yellow mustard meal | 47.1 | 53.5 | 28 | 7.9 | 226.9 | 63.3 |
| Oriental mustard meal | 46.7 | 56.2 | 51.6 | 3.9 | 245.9 | 61.5 |
| Brown mustard meal | 43.2 | 54.3 | 52.3 | 4.1 | 230.7 | 60.6 |
| Soybean flour (commercial) | 52.4 | 67.7 | 58.1 | 58.6 | 196.7 | 51.9 |
| PS: Protein solubility; EAI: Emulsifying activity index; ES: Emulsion Stability; FC: Foaming capacity; FS: Foaming stability | ||||||
Mustard protein concentrates or isolates can be used at 2% in processed meat products, at 1% in bakery products, nutritional bars, soups, beverages, infant formula, as a nutritional supplement or as a replacement for other proteins (e.g. gluten, casein and soy proteins). Mustard proteins exhibit excellent fat and water binding properties, gelling and emulsion stabilizing properties. Examples of food applications include:
- Soluble mustard proteins isolates, being acid soluble, could be used to enrich carbonated soft drinks or non-carbonated acidic fruit drinks at a (0.2 to 5% level)7
- The functionality of Oriental mustard meal and derived protein isolates in meat emulsions (wiener and bologna) was found to be similar to that of soy protein isolate in terms of colour, texture and flavor, as ranked by a sensory test panel9
- Mustard protein isolate, prepared by steam injection, has been used to fortify pasta with increased protein content and protein digestibility1
- A calcium soluble protein fraction from Yellow mustard seed meal has potential application as an additive to prepare calcium-fortified high protein liquid products4
Mustard Protein Allergenicity and Labelling
It is the napin proteins in Brown (Bra j 1) and Yellow (Sin a 1) which are most likely associated with mustard’s allergenicity.8,10 Mustard, like wheat and soy, is considered a priority allergen in Canada and Europe and like these other ingredients must be identified as a food allergen in the food label ingredient list. The food industry, through proper labelling, can adequately inform consumers.
New Mustard Protein Application Opportunities
- Capitalize on the properties of mustard seed protein such as: i. its solubility over various pH ranges, ii. emulsifying and foam forming properties, and iii. heat induced gelation properties. These can be applied over a wide selection of food products and perhaps replace or complement soy and dairy proteins in food applications
- Focus on functional and nutritional ingredient properties to produce high value protein ingredients for inclusion in consumer food and nutraceutical products, feed products and industrial products (adhesives, glues, foams)
- Differentiate the unique, inherent properties of Yellow or Brown/Oriental proteins and modify them via phosphorylation, acylation, or glycosylation to maximize value
- Explore hydrolysis to generate bioactive peptides with unique nutritional properties or health benefit
References
- Alizera-Sadeghi, M. and Bhagy, S. 2008. Food Sci. 73:S229-S237
- Aluko, R. E. and McIntosh, T. 2004. Am. Oil Chem. Soc. 81:679-683
- Aluko, R. E., Reaney, M., McIntosh, T., Ouellet, F., and Katepa-Mupondwa, F. 2004. Agric. Food Chem. 52:6030-6034
- Aluko, R.E., McIntosh, T., Katepa-Mupondwa, F. 2005. Sci. Food Agric. 85:1931-1937
- Bos, C., Airinei et al., 2007. J. Nutr. 137:594-600
- Cavins, J.F., Kwolek, W.F., Inglett, G.E. and Cowan, J.C. 1971. Assoc. Cereal Chem. Annual Meeting, October 1971. Dallas, TX
- Diosady, L.L. Xu, L. and Chen, B.-K. 2011. US Patent 8048463
- Marambe, H.K., McIntosh, T.C., Cheng, B., and Wanasundara, J.P.D. 2014. Food Control 44:233-241
- Marnoch, R. and Diosady. L.L. 2006. JAOCS 83:65-69
- Monsalve, R. I., Villalba, M., and Rodrıguez, R. 2001. Symp. Food Allergens 3:57–69
- Terras, F. R. G., Torrekens, S., Van Leuven, F., Osborn, R.W., Vanderleyden, J., Cammue, B.P.A., Broekaert, W.F. 1993. FEBS Lett. 316:233–240
- Wanasundara, J.P.D. and McIntosh, T.C. 2013. US Patent 8,557,963
- Wanasundara, J.P.D. 2011. Rev. Food Sci. Nutr. 51:635-677

